KOCITAF as Emergency PEP to prevent HIV infection in Bangkok, Pattaya, Phuket, Chiang Mai - Thailand
19413
KOCITAF as Emergency PEP to prevent HIV infection in Bangkok, Pattaya, Phuket, Chiang mai - Thailand

Condom broke in Thailand | Emergency PEP
KOCITAF as Emergency PEP to prevent HIV infection in Bangkok, Pattaya, Phuket Chiang Mai - Thailand
To order Kocitaf - Dolutegravir, Emtricitabine & Tenofovir Alafenamide HIV Treatment Medication
For more information about the medication and ordering process,
please email pulseliving@pulse-clinic.com or chat on your preferred platform.
![]() +66-84-226-2569
+66-84-226-2569  @pulserx
@pulserx ![]() PulseClinic
PulseClinic
A new anti-HIV drug has arrived in Thailand
The combination anti-HIV drug Kocitaf was now approved by the Food and Drug Administration of Thailand on February 3, 2021, and is intended for the treatment of adults and adolescents (aged 12 years and over and has It is hoped that the government will soon allow people living with HIV to receive the drug with gold patents and social security. What are the advantages and disadvantages of this drug compared to the current popular formulation (Teevir brand)? First of all, I would like to present a fairly academic content so that the reader can gain a rather in-depth knowledge of the drug.
Kocitaf contains
- Tenofovir alafenamide fumarate (TAF) 25 mg
- Emtricitabine (FTC) 200 mg
- Dolutegravir (DTG) 50 mg
Introduction to the division process of the HIV virus
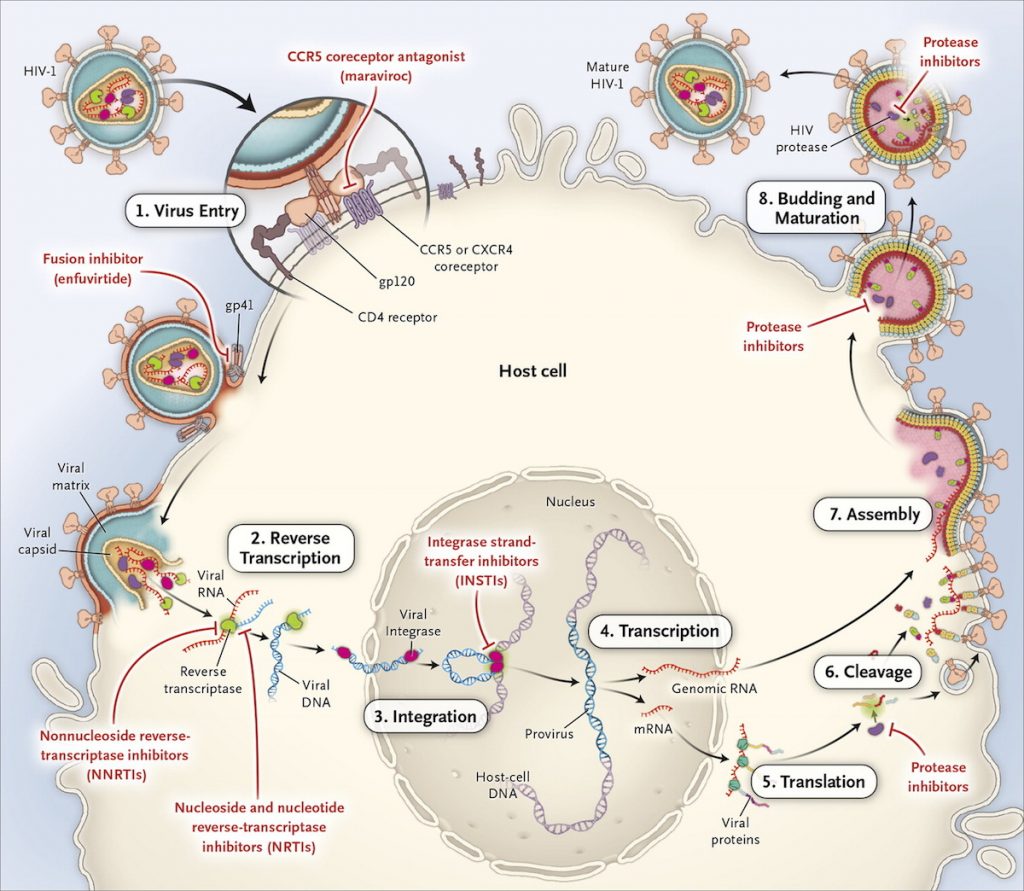
antiretroviral drugs used to treat people with HIV Multiple combinations are required. This is because the HIV virus is complex in the process of reproduction. HIV is a retro virus, observed by its genetic transcription process, from the underlying RNA to DNA, which appears to be a reverse process. It relies on the resources of white blood cells to regenerate itself, which consists of several steps. both within the nucleus and other elements of the cell Therefore, a single drug is not enough to inhibit the replication of the virus. The rough process of virus propagation is as follows (1) (shown in Figure 1).
Attaches to the cell wall and enters the cell (Virus entry)
- Transcription RNA to make viral DNA (Reverse Transcription)
- into the nucleus, its DNA is inserted into the DNA of the cell (Integration)
- DNA strands are removed as mRNA and Genomic RNA and leave the nucleus. (Transcription)
- Viral mRNA is decoded to synthesize proteins to make up new viral components. (Translation)
- The protein strands are cut into tiny particles to prepare for cleavage. (Cleavage)
- Genomic RNA and synthesized proteins make up several new viruses (Assembly)
- The new virus prepares to leave the cell wall intact (Budding and Maturation)
Add us on Line and stay in touch.
A new drug of choice for emergency PEP and treating people with HIV
Kocitaf contains two non-nucleoside reverse transcriptase inhibitors (NNRTIs), TAF and emtricitabine. Combined with one Integrase strand transfer inhibitor (INSTIs), Dolutegravir.
by TAF and Emtricitabine It inhibits HIV viral replication in Process 2 (Reverse transcription) and Dolutegravir inhibits Process 3 (Integration).
The 2017 Thailand HIV Treatment and Prevention Guidelines state that TDF is the basic antiretroviral drug. It is most commonly used as a first-line treatment. (2) In general, for treatment, TDF is usually taken as one tablet (300 mg) once a day in combination with other antiretrovirals. However, this dosage was quite high. which the drug recipient must take every day continuously Therefore, they are more likely to experience side effects from the use of this drug, such as nephrotoxicity and bone marrow, which requires regular monitoring of kidney function.
Tenofovir Alafenamide fumarate (TAF) is a new drug that is gaining attention. It can help reduce side effects on kidneys and bones by using doses much lower than TDF.
Dolutegravir (DTG)
It is a second-generation integrative inhibitors and was approved by the U.S. Food and Drug Administration (FDA) in 2013 for its high resistance to HIV virus. Tivicay 10, 25, and 50 mg single tablets are available, and Triumeq combined tablets contain Abacavir 600mg, Lamivudine 300mg, Dolutegravir 50mg.
The pharmacokinetics of Dolutegravir is more than 98.9% bound to blood proteins. Its volume of distribution is 17.4 L. The plasma half-life is 14 hours. The drug is metabolized via UGT1A1 and partially via the CYP3A enzyme. It affects CYP3A. When used with dolutegravir, it also affects the level of dolutegravir in the body. For example, antacids or multiionic drugs can decrease dolutegravir levels. The drug is eliminated in the stool by 53%.
It was found that the food taken with this drug increase the amount of drug absorption but reduces the rate of drug absorption But it is not clinically insignificant. Therefore, this drug may or may not be taken with food.
Interactions between dolutegravir and metformin (for diabetes) were found to increase Metformin levels 2.4-fold and peak Metformin 2-fold.
Dolutegravir plus Rifampicin (tuberculosis treatment) has been found to decrease dolutegravir by 54%. Therefore, dolutegravir 50 mg originally taken once a day may need to be increased to twice a day.
Dolutegravir side effects such as insomnia, headache, and depression can occur in patients with a history of psychiatric problems. Pregnant women should be careful Especially at less than 8 weeks of gestation, if you have not started antiretroviral therapy. It is advisable to start with another antibody formulation instead. If you are over 8 weeks pregnant, you can receive Dolutegravir under close monitoring by your doctor.
New recommendation from WHO
The World Health Organization has released an Update of recommendations on first- and second-line antiretroviral regimens Policy brief 22 July 2019, recommending Dolutegravir over Efavirenz due to less drug–drug interactions, faster viral suppression, higher genetic barrier to drug resistance Although dolutegravir may cause weight gain, the issue of neural tube defects in infants in Botswana is unclear. and from the study compare with efavirenz-based No differences were found, including the incidence in other countries did not increase.
KOCITAF is available for emergency HIV prevention (Post-exposure prophylaxis)
and for HIV treatment at PULSE CLINIC throughout Thailand.
To order Kocitaf - Dolutegravir, Emtricitabine & Tenofovir Alafenamide HIV Treatment Medication
For more information about the medication and ordering process,
please email pulseliving@pulse-clinic.com or chat on your preferred platform.
![]() +66-84-226-2569
+66-84-226-2569  @pulserx
@pulserx ![]() PulseClinic
PulseClinic
Chat with PULSE CLINIC ONLINE Department
Loading...
Clinic Locations
Loading...